
- #ALKENE HYDROCARBON WITH 6 CARBONS AND DOOBLE BONDS HOW TO#
- #ALKENE HYDROCARBON WITH 6 CARBONS AND DOOBLE BONDS FULL#
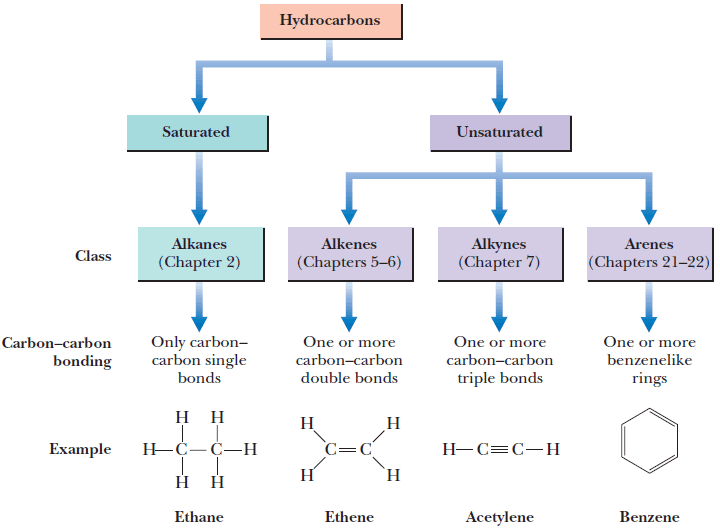
In my opinion, the most industrially important alkenes are the vinyl, cis-, trans-, and trisubstituted varieties. Lastly, a C=C bond with four R-groups is called a tetrasubstituted alkene, and again has no structural isomers. When an alkene contains three R-groups and one hydrogen it is called a trisubstituted alkene. As we will see below, cis and trans isomers have different IR spectra, which is why IR spectroscopy can be used to distinguish and quantitate these fats in samples. Many food labels these days contain statements about how much cis and trans fats a product contains. The prefix cis is also derived from a Latin word and means “on the near side.” The cis and trans isomers of fats have been in the news because of their effect on human health. One way of remembering these terms is that the Latin word “trans” means “across,” and that the English verb “to transport” means to carry something across from one place to another. As illustrated in Figure 1, cis isomers have their hydrogens on the same side of the double bond, while trans isomers have their hydrogens diagonally across from each other. The other two types of C=C bonds with two hydrogens are called cis and trans.

Vinylidene molecules are not common industrially and will not be discussed further. In a vinylidine molecule the two hydrogens are attached to one carbon while the R-groups are on the other carbon. There are three different alkene structural isomers that consist of a C=C bond with two hydrogens and two R-groups attached.
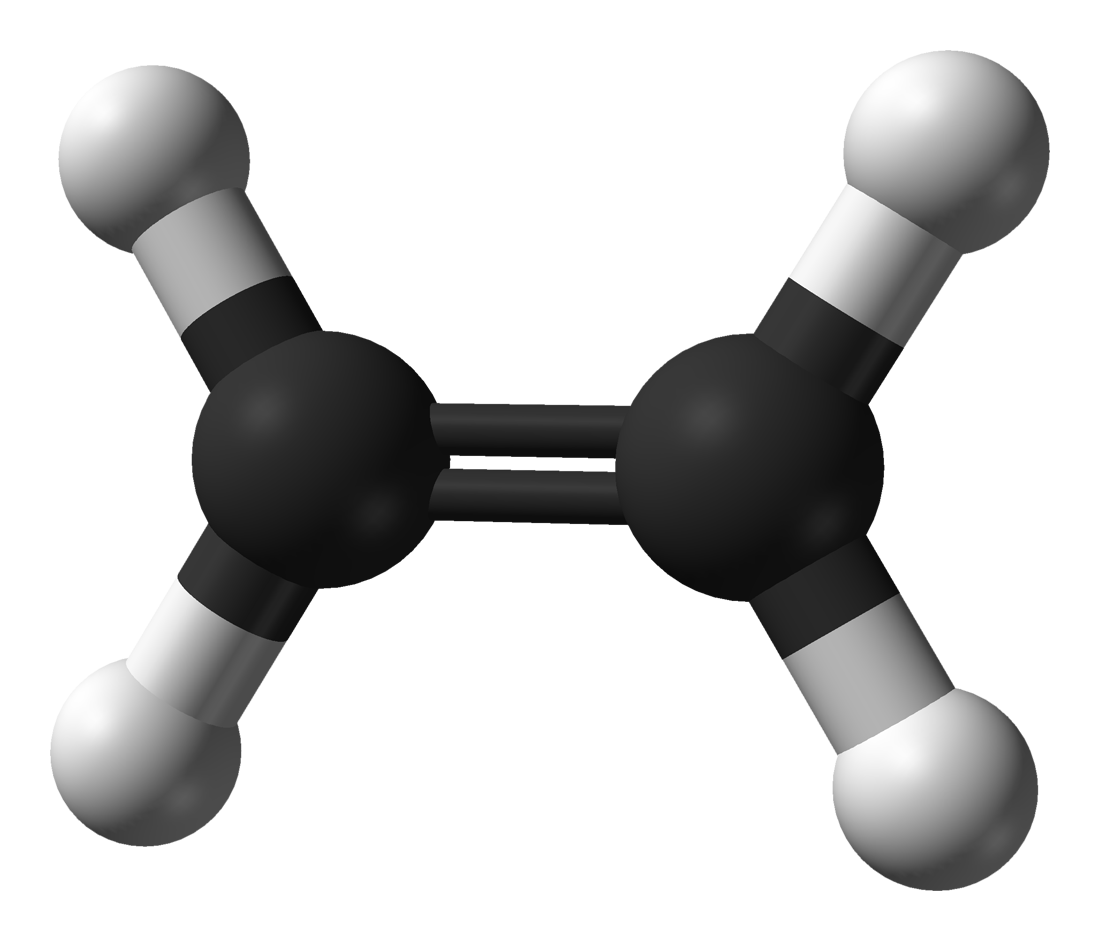
Vinyl groups consist of a double bond with three hydrogens and one nonhydrogen atom attached, which will be referred to henceforth as “R-groups.” Vinyl groups are important industrially because they react to form common polymers such as polypropylene, polystyrene, and polyvinylchloride. A diagram of the different types of alkenes is shown in Figure 1. Alkenes consist of six different varieties, some of which exhibit structural isomerism. IR spectroscopy can distinguish between structural isomers because these molecules have different bond strengths, reduced masses, and hence different peak positions (5). Structural isomers are molecules that have the same chemical formula but different chemical structures, such as the ortho, meta, and para isomers of disubstituted benzene rings discussed previously (4). Hydrogenated vegetable oil contains unsaturated fats whose C=C double bonds have been hydrogenated to form saturated fats. The Chemical Structure of AlkenesĪlkenes contain unsaturated carbons because C=C bonds can have hydrogens added to them via a reaction known as hydrogenation to create saturated carbons (3). Going forward, you can simply assume that the peak position units will be in wavenumbers (cm -1) although the text will not say as such. This column focuses on using infrared (IR) spectroscopy to distinguish the different types of alkenes from each other.Īs an aside, starting with this column installment I will stop using cm -1 after every peak position. This example shows that benzene and other aromatic rings contain unsaturated carbons.Īnother family of unsaturated hydrocarbons contain carbon–carbon double bonds, C=C, and are called alkenes.
#ALKENE HYDROCARBON WITH 6 CARBONS AND DOOBLE BONDS FULL#
Unsaturated hydrocarbons can have hydrogens attached to them via chemical reaction, which means these molecules do not have their full theoretical complement of hydrogens and hence are “unsaturated” with respect to hydrogen substitution (3).įor example, aromatic rings have a carbon–carbon bond order of about 1.5 (3), and the prototype aromatic compound benzene, with the chemical formula C 6H 6, can have hydrogens added to it to form the saturated compound cyclohexane, C 6H 12 (3). These hydrocarbons contain functional groups with carbon–carbon bond orders > 1. Before that, I discussed how aromatic rings are a type of a larger class of molecules called unsaturated hydrocarbons (2).
#ALKENE HYDROCARBON WITH 6 CARBONS AND DOOBLE BONDS HOW TO#
We are done, for the time being, with our discussion of how to distinguish the isomers of mono- and disubstituted benzene rings from each other, which centered in part on the infamous “benzene fingers” (1). This column provides you with all the tools you need to distinguish all of these different types of molecules from each other. C=C bonds, otherwise known as alkenes, come in six different structural isomer types.

Now that we have completed our discussion of benzene rings and the infamous “benzene fingers,” the next topic on our hydrocarbon hit parade is carbon–carbon double bonds.


 0 kommentar(er)
0 kommentar(er)
